This method is based on electrolysis. Hence the option A is incorrect.

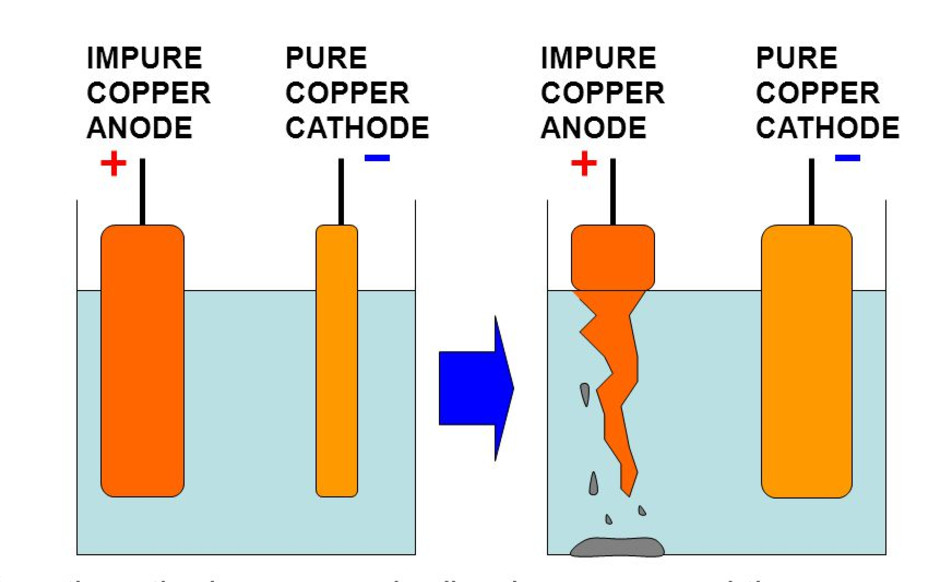
The Diagram Shows The Process Of Purification Of Copper Metal A Thick Copper Plate A Of Impure Copper And A Thin Copper Plate B Are Immersed In A Metal Sulphate Solution And
Hence the option B is correct.
. When the molten metal is stirred with green wood poles the impurities come to the surface get oxidised and form a scum which can be removed. Ii heating with iodine. Purification of ores Another application of electrolysis is in the purification of metals such as copper.
Wood gases hydrocarbons from wood reduce any oxide of the metal back to the metal. Semiconductors such as S i G e G a are purified by this process. Liquation method is used for the purification of A copper B iron C aluminium D lead The correct answer is D Lead Liquation Metals with low boiling points are made to flow on a sloped surface thereby separating them from the impurities which have higher boiling points.
Given the atomic mass. For example the main ores of copper are mixtures of copper and iron oxides and copper production from the ore is carried out by electrolytic extraction. B On passing electric current metal ions from the electrolyte are deposited at the cathode in the form of pure metal.
Electrolystic refining or electrorefining. B The electrolyte used is acidified aqueous copper sulphate solution. Lead tin and bismuth are purified by the liquation method.
Discuss the importance of electrolysis in the smelting and purification of metals. For example - S n O in impure S n O 2 and C u 2 O in blister copper. A Crude copper is made anode a thin sheet of pure copper is made cathode and acidified solution of copper sulphate is used as an electrolyte.
The copper sulfate solution the pregnant leach solution is then stripped of copper via a solvent extraction and electrowinning SX-EW plant with the barred denuded sulfuric acid recycled back on to the heaps. This method is used to refine copper Cu silver Ag gold Au lead Pb aluminium Al platinum Pt. It is typically used to purify metals like copper or tin that are in the impure form of copper oxide or tin oxide.
In the extraction of copper from its sulphide ore the metal is finally obtained by the reduction of cuprous oxide with Copper I sulphide Cu 2 S sulphur dioxide SO 2. How much copper metal in grams could be produced from copperII oxide by applying a current of 100 amps at the appropriate negative potential for 120 hours. Expert Solution Want to see the full answer.
Copper and tin are purified by this process. The vapours of the pure metal are then condensed leaving the impurities behind. Copper is refined using an electrolytic method.
Add few drops of dilute sulphuric acid to copper sulphate solution. This method is used for the purification of metals that possess a low boiling point such as mercury and zinc. The electrodes are immersed in a CuSO 4 solution.
The sulfides are most abundant and yield copper metal when heated. I heating with stream of carbon monoxide. During purification of copper by electrolytic refining of blister copper A Impure Cu strip blister copper is used as anode whereas pure Cu strip is used as cathode.
In this method crude metal is made anode whereas the thin sheet of pure metal is made cathode. Electrical refinement of copper is the process within which copper is subjected to electrolysis inside a cell. The method used for purifying the ores is by electrolytic extraction.
Check out a sample QA here See Solution Want to see the full answer. The cathode segment of this cell is pure copper and the anode segment is impure copper along with other metals. B Zone refining This method is employed for preparing highly pure metal such as silicon tellurium germanium which are used as semiconductors.
Copper metal 63546 gmol is purified by electrolysis. When copper is made from sulphide ores by the first method above it is impure. Poling is a method used to purify metals that have oxidized impurities.
In the electrorefining process the ore is dissolved in an electrolyte and this solution is electrolyzed. This method is used when the impure metal contains impurities of its own oxide. Some impurities have greater affinity for oxygen than for the metal.
The more reactive metals like iron that are present in the crude copper are. This method may seem strange but in order to do this we take a log of wood that is. The anode is made of the impure Cu electrode and the cathode is the pure Cu electrode.
Copper metal can be purified electrolytically according to the following arrangement. Its typically used to purify metals like copper or tin that are in the impure form of copper oxide or tin oxide. The impurities do not vaporize and hence they are separated.
It is based on the principle that melting point of a substance is lowered by the presence of impurities. To purify impure copper metal Take 250 ml of distilled water in a clean beakerDissolve two teaspoon full of copper sulphate in it. This method is based upon the principle of fractional crystallisation.
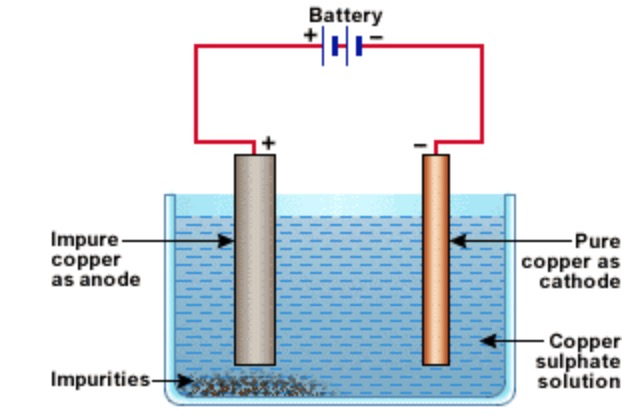
Copper processing is a complicated process that begins with mining of the ore less than 1 copper and ends with sheets of 9999 pure copper called cathodes which will ultimately be made into products for everyday useThe most common types of ore copper oxide and copper sulfide undergo two different processes hydrometallurgy and pyrometallurgy respectively due. A thick rod of impure copper metal is made positive electrode by connecting it to the positive terminal of the battery. In which of the following method of purification metal is converted to its volatile compound which is decomposed to give pure metal.
Purification of copper by electrolysis Cu s Cu 2 aq 2e - at anode Cu 2 aq 2e Cu s at cathode Therefore 9995 pure copper is obtained in this procedure. Most copper is mined in the form of the ores chalcopyrite CuFeS2 chalcocite CU2S and malachite Cu2C030H2. The blister copper is first treated to remove any remaining sulphur trapped as bubbles of sulphur dioxide in the copper - hence blister copper and then cast into anodes for refining using electrolysis.
Poling is a method used to purify metals that have oxidized impurities. The most important electrochemical reaction that occurs in the course of copper electrolysis follows Faradays first law. The molten impure metal is stirred with green wood poles.
It is used to purify copper. Copper is refined to 9998 pure copper by electrolytic refining Fig. Processes of isolation of elements.
In this process the impure metal is heated above its boiling point so that it can form vapours.
Purification Or Refining Of The Metal Chemistry Class 12 General Principles And Processes Of Isolation Of Elements

Purification Of Metal Chemical Effect Of Electric Current Class 8

0 Comments